-
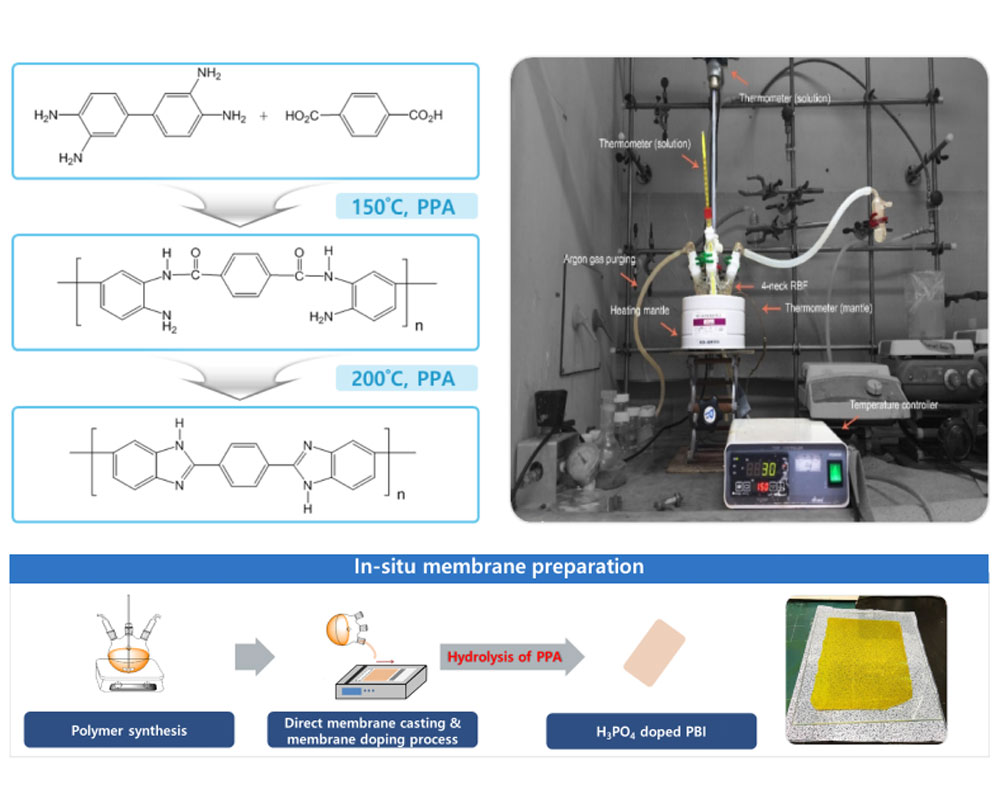
- I. Synthesis of polybenzimidazoles for high temperature PEMFC
-
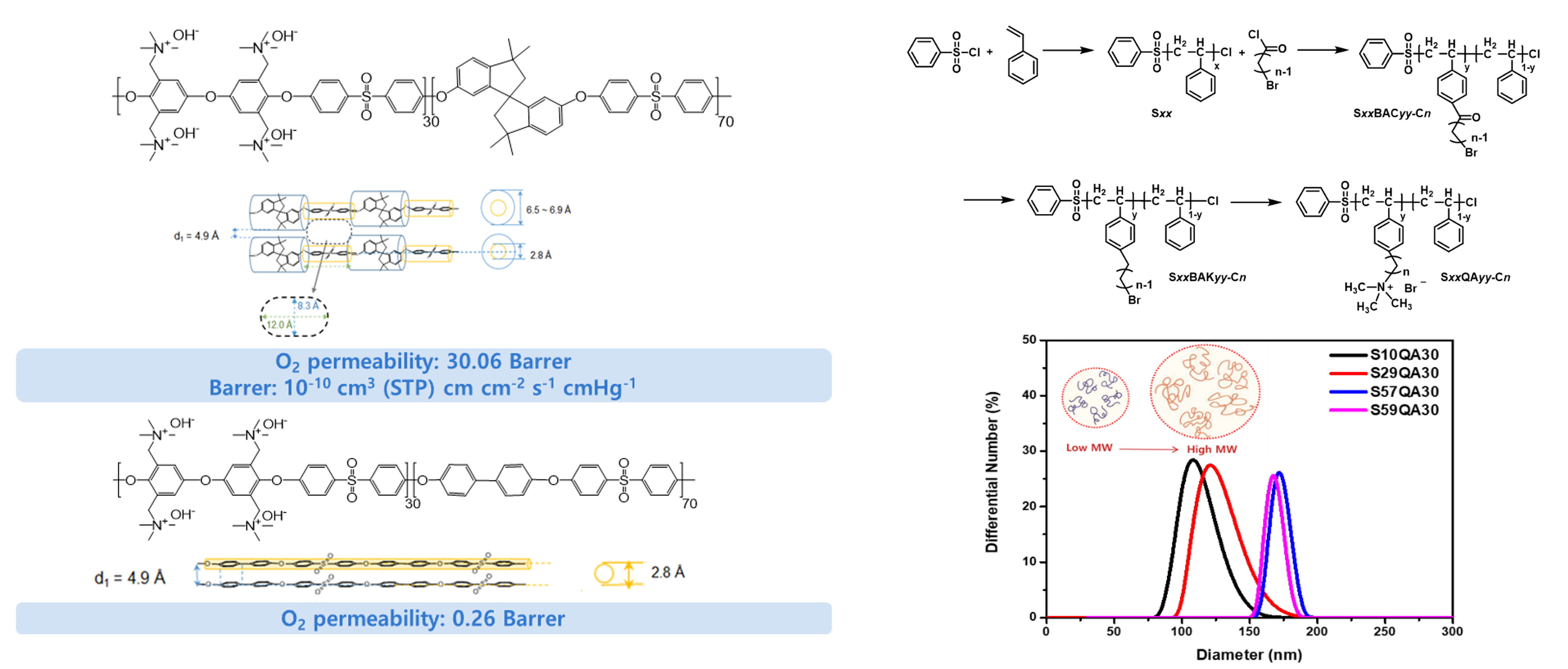
With the growing demand for clean energy conversion systems, PEMWEs and PEMFCs are being developed as promising alternatives to fossil fuels as energy generation systems. Among the various components of PEMWEs and PEMFCs, proton exchange membranes (PEMs) are very important materials that determine their overall performance. The most widely used PEMs are perfluorinated sulfonic acid (PFSA) polymer membranes, such as Nafion®, which exhibit superior properties, including high proton conductivity and durability in a temperature range of 10–80 °C. However, these materials suffer several drawbacks, such as their high cost, complex synthetic protocols, and recent environmental and regulatory issues associated with polyfluoroalkyl substances.
To replace Nafion®-type PFSA PEMs, I have developed hydrocarbon-based sulfonated PEMs, which offer superior stability, high proton conductivity, and low-cost production. In particular, I have focused on developing technologies that can be applied practically in industry. I will continue to develop low-cost, high-performance PEMs for PEMWEs and PEMFCs, and devote considerable effort to leading the early commercialization of these energy conversion technologies.
-
- II. Gas permeable binder for electrodes of anion exchange membrane (AEM) fuel cells and electrolyzers
-
Recently, the development of water electrolyzers that produce hydrogen using renewable energy is being actively pursued. Studies on fuel cells capable of utilizing the hydrogen produced by water electrolyzers most efficiently are also being conducted. Hydrogen energy conversion technologies, such as electrolyzers and fuel cells, provide alternatives to fossil-fuel-based energy production, offering meaningful solutions to the current issues surrounding environmental impact and resource depletion.
Hydrogen energy conversion technologies based on proton (H+) conduction mechanisms, such as PEMFCs and PEMWEs, require the use of Nafion®-type PFSA electrolyte membranes, platinum group metal (PGM) catalysts, and oxidation-resistant porous transport media. As the supply of renewable energy increases, the demand for hydrogen production and utilization will also increase. Therefore, the high cost of the materials required for PEMFC and PEMWE manufacture will become a significant drawback. Many researchers are focusing on finding ways to lower the price of these materials, but they have not yet produced significant results.
Instead, I have been studying a technology that produces and utilizes hydrogen employing a mechanism that delivers hydroxide ions (OH-) rather than focusing on a proton conduction mechanism. SAMWEs and SAMFCs, which operate based on an OH- delivery mechanism, have the advantage of using low-cost electrolyte membranes and transition metal catalysts for energy conversion.
-
- III. Development of self-humidifying dual exchange membrane fuel cells (DEMFCs)
-
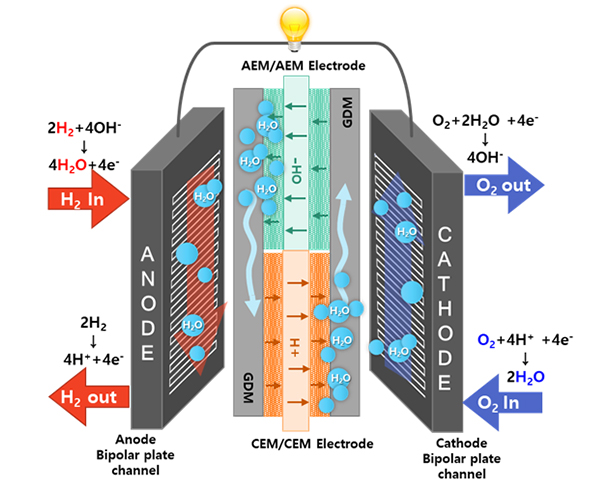
The miniaturization of fuel cell systems is crucial for developing fuel cells for drones and unmanned aerial vehicles (UAVs). PEMFCs are generally used by these aircraft. In general, a humidifier is located next to the PEMFC because humidified fuel and air must be supplied to achieve high performances. Because the humidifier in fuel-cell-powered aircraft increases the weight and volume, it is necessary to develop a PEMFC that exhibits excellent performance without a humidifier. I developed a new-concept self-humidifying dual exchange membrane fuel cell (DEMFC) to overcome the limitations of the PEMFC.
The DEMFC was fabricated using sequentially aligned MEAs consisting of an anion exchange membrane (AEM) and a cation exchange membrane (CEM). The AEM and CEM MEA sections in the DEMFC are expected to be mutually humidified to enable non-humidified operation. On the anode side, dry hydrogen supplied to the AEM MEA section is electrochemically oxidized by hydroxide ions to produce water. The water generated at the anode of the AEM MEA section is transported through the gas diffusion medium (GDM) to humidify the anode and promote the HOR in the CEM MEA section. Meanwhile, on the cathode side, dry oxygen supplied to the CEM MEA section is electrochemically reduced by protons to produce water. Similarly, the water generated at the cathode of the CEM MEA section is supplied to the AEM MEA section to enhance the ORR. Currently, there are no fuel cells that generate water at the anode and cathode. For the first time, I have developed a new type of fuel cell that produces water at both the anode and cathode, allowing for the elimination of an external humidifier in a fuel cell system. The use of this technology is expected to facilitate fuel cell water management in the future.
-
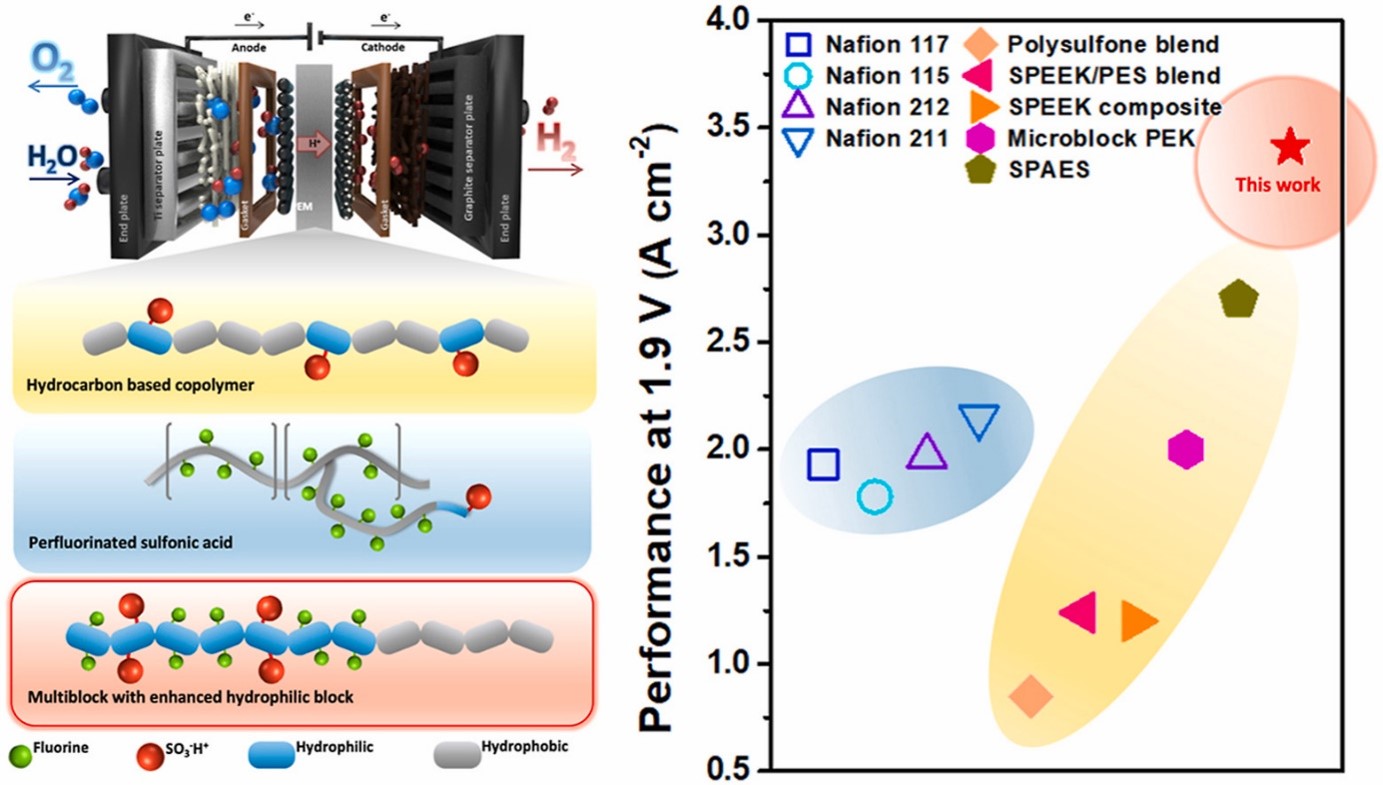
- IV. Hydrocarbon-based polymer electrolyte membrane for proton exchange membrane (PEM) water electrolyzers
-
Hydrocarbon membranes are potential alternatives to Nafion in proton exchange membrane water electrolysis (PEMWE). These membranes are composed of hydrocarbon-based materials, such as aromatic and aliphatic polymers, which offer several advantages over Nafion. One advantage is their cost-effectiveness compared to Nafion membranes. Hydrocarbon membranes are typically less expensive to produce, making them more economically viable for large-scale PEMWE applications. Additionally, hydrocarbon membranes exhibit good chemical stability and mechanical strength, which contribute to their durability and long-term performance. They can withstand the harsh operating conditions of PEMWE, including high temperatures and acidic environments. Our lab is actively working to enhance its proton conductivity, water management capabilities, and overall performance to meet or exceed the standards set by Nafion membranes.
-
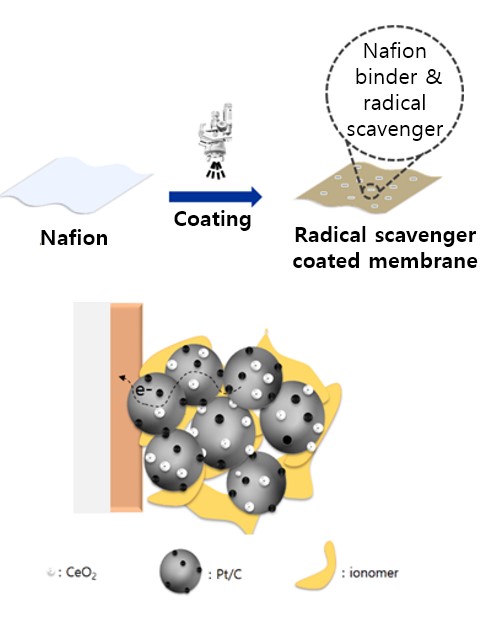
- V. Application of new radical scavengers to membrane electrode assembly (MEA) fabrication
-
As part of the membrane electrode assembly (MEA) fabrication process, radical scavengers can be added to increase the durability and stability of the MEA. The radical scavengers react with free radicals, which are highly reactive and can damage MEA. Adding radical scavengers to the MEA fabrication process can enhance the long-term durability and stability of the fuel cell, especially under harsh operating conditions where free-radical formation is more prevalent. In order to achieve the desired improvements in durability and stability without compromising overall fuel cell performance, radical scavenger concentration and type should be carefully evaluated and optimized during MEA fabrication.
As a lab that has a Fuel Cell Station, a combination of experiments and modeling tests is used to incorporate radical scavengers into MEA fabrication processes.